Non-CDS Medication Data
Reporting of Non-Controlled (Non-CDS) Medication Data
Reporting of Non-Controlled (Non-CDS) Medication Data

The Maryland Health Care Commission (MHCC) and CRISP have begun collection of non-controlled substances (“non-CDS”) in accordance with Maryland House Bill 1127 Public Health – State Designated Exchange – Health Data. CRISP, in partnership with Leap Orbit, has built an infrastructure to receive, process, store and deliver non-CDS data. Dispensers were required to begin reporting non-CDS data starting September 1st, 2025.
Through the contract between CRISP and Leap Orbit, dispense data is securely collected and stored using RxGov. This is a web-based program that does not require additional hardware or software. Dispense data available for a given patient will be visible to CRISP clinical users under the Medication Management tab of InContext and Clinical Information, within the new Dispensed Medications subtab.
As the state designated HIE/HDU, CRISP is responsible for collecting non-controlled medication data
Per CRISP policy, submitters are required to sign one of two agreements:
Note: If submitters have sufficient reason why they are not able to submit right now, they can submit a waiver for the year through MHCC. More information available on the MHCC website. If a waiver is accepted, no agreement is required
If you want to sign a Participation Agreement (PA) or Connection Agreement (CA):
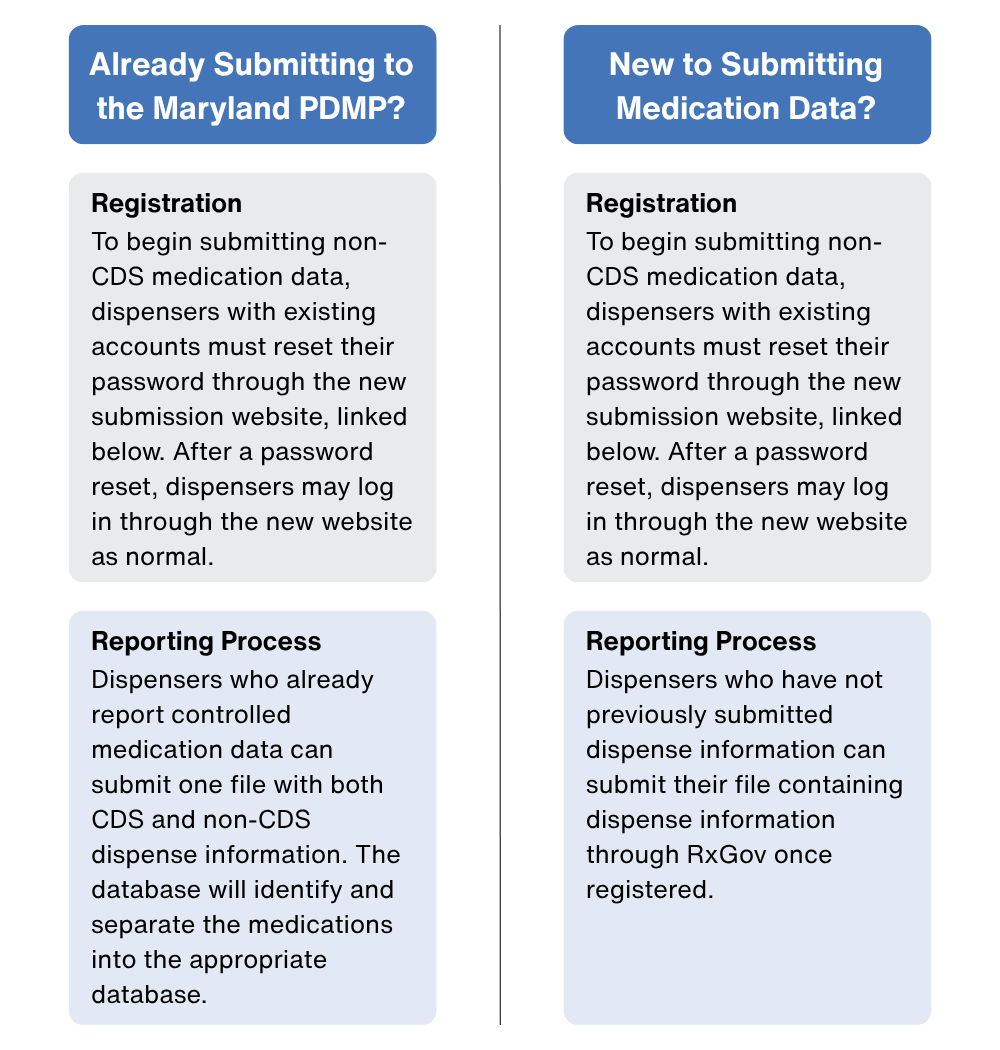
| Current Submitters of Controlled Data | New Submitters of Medication Data |
|---|---|
Registration: | Registration: |
Reporting Process: | Reporting Process: |
Through this new infrastructure, CRISP clinical users will be able to review data on all medications a patient has received – not just controlled substances. This will help providers stay properly informed about their patient when prescribing and treating. CRISP will display noncontrolled medication data as part of the standard clinical data. This ensures medication information is easily accessible next to other medical data, providing a full view of a patient’s treatment.
All organizations seeking clinical access must sign a Standard Participation Agreement with CRISP before getting started.

If you have already signed a Participation Agreement and still need clinical access, please contact CRISP at support@crisphealth.org and we will work with you to ensure that individuals at your organizations receive appropriate access.
More detailed information on getting connected with us is available on our onboarding webpage.
Dispensed medication data will be available through the Clinical Information Service and the InContext Application. To view dispensed medication information available for a given patient:
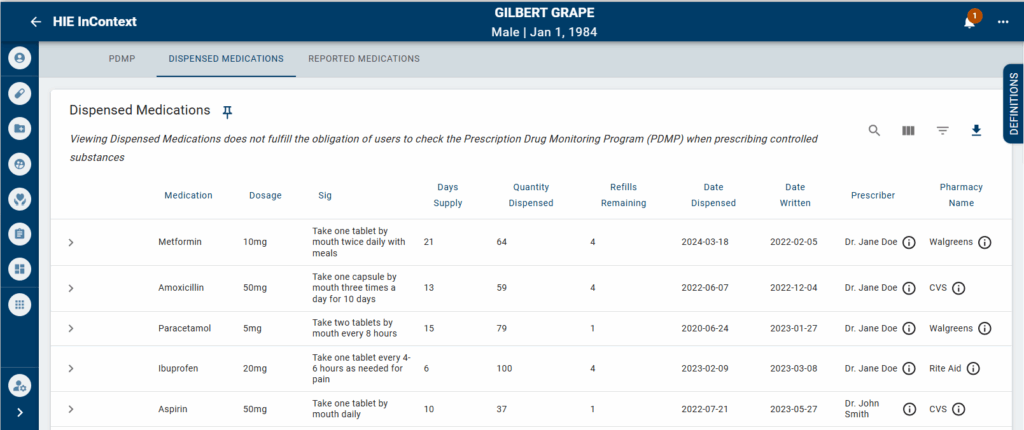
When viewing medication data, there are two different tabs data could fall under: Reporting and Dispensed Medications.
Subscribe